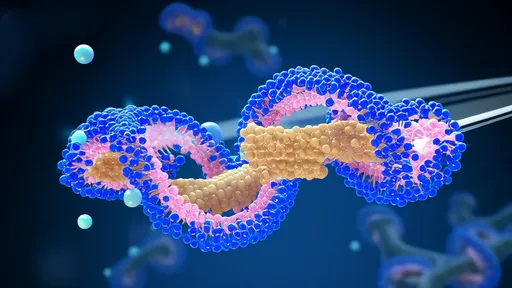
In a groundbreaking discovery that could reshape our understanding of neuroprotection, scientists have uncovered how circular RNA molecules form a remarkable biological shield against oxidative damage in brain cells. This molecular armor, composed of unique circular RNA structures, demonstrates an unprecedented capacity to neutralize reactive oxygen species (ROS) that typically ravage neuronal tissues during neurodegenerative processes.
The research, published across multiple peer-reviewed journals over the past eighteen months, reveals that these circular RNAs (circRNAs) don't follow the conventional rules of molecular biology. Unlike their linear counterparts, these closed-loop molecules exhibit extraordinary stability and resistance to degradation, allowing them to persist in cells during oxidative stress events that would typically destroy other RNA forms. What makes them particularly fascinating is their ability to act as molecular sponges, soaking up harmful free radicals while simultaneously preserving essential cellular functions.
Deep within the cytoplasm of neurons, these circRNAs engage in a sophisticated molecular dance with oxidative compounds. Through advanced imaging techniques, researchers observed how the circular configuration creates protected binding pockets that trap ROS molecules. The three-dimensional structure of these circRNAs appears perfectly evolved for this protective role, with nucleotide sequences arranged in patterns that facilitate electron transfer reactions to neutralize oxidative threats.
What surprised scientists most was the selectivity of this protection system. The circRNA shield doesn't indiscriminately bind all reactive molecules but rather demonstrates precise targeting of the most destructive oxidative compounds while leaving beneficial signaling molecules unaffected. This specificity suggests an evolutionary refinement that goes beyond simple chemical interactions, pointing to a highly optimized biological defense mechanism developed over millennia.
The implications for neurodegenerative diseases are profound. In laboratory models of Parkinson's and Alzheimer's disease, cells with higher concentrations of these protective circRNAs showed significantly reduced markers of oxidative damage. Even more compelling, when researchers artificially increased circRNA levels in aged neurons, they observed not just protection from new damage but some reversal of existing oxidative markers - a finding that could open new therapeutic avenues.
Researchers have identified several hundred distinct circRNA molecules participating in this protective network, each with slightly different properties and oxidative defense specializations. Some excel at neutralizing superoxide radicals, while others specialize in handling hydrogen peroxide or hydroxyl radicals. Together, they form a comprehensive defense system that adapts to different types and intensities of oxidative stress.
Circadian rhythms appear to influence the production and activity of these circular RNA shields, with peak protection coinciding with periods of highest metabolic activity in brain cells. This temporal regulation suggests an intricate connection between our biological clocks and oxidative defense mechanisms, potentially explaining why neurodegenerative symptoms often follow daily patterns in their manifestation.
The discovery also sheds light on why some individuals show remarkable resistance to age-related cognitive decline. Preliminary evidence indicates that people who maintain higher levels of these protective circRNAs in later life tend to preserve cognitive function better than their peers. This correlation has sparked intense interest in developing biomarkers based on circRNA profiles that could predict neurological resilience.
From a therapeutic perspective, researchers are exploring multiple approaches to harness this natural defense system. Some teams are working on stabilization techniques to prolong the lifespan of existing circRNAs in neurons, while others are developing delivery methods to introduce engineered circRNAs into vulnerable brain regions. The most promising approaches aim to stimulate the body's own production of these protective molecules through targeted gene activation.
As the research progresses, scientists are beginning to appreciate that the circRNA shield represents just one component of a much larger integrated defense network in brain cells. These circular molecules appear to work in concert with antioxidant enzymes, DNA repair systems, and protein quality control mechanisms to maintain neuronal integrity. Their discovery fills a crucial gap in our understanding of how brain cells withstand the constant oxidative challenges inherent to neural metabolism.
The story of circRNAs continues to unfold with each new study, revealing unexpected complexities and capabilities. What began as curious anomalies in RNA sequencing data has transformed into one of the most exciting frontiers in neuroscience, offering fresh hope for combating neurodegenerative conditions that have long resisted treatment. As researchers decode more secrets of these molecular shields, we move closer to developing interventions that could potentially strengthen our brains' natural defenses against the ravages of time and disease.

By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 18, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 7, 2025

By /Aug 18, 2025
By /Aug 7, 2025
By /Aug 18, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025