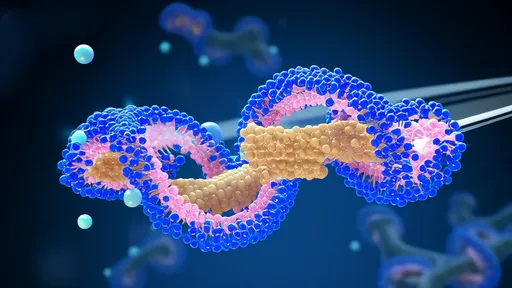
In a groundbreaking discovery that bridges quantum physics and cell biology, researchers have unveiled a startling new mechanism for ion transport across cell membranes. Dubbed the "quantum express" system, this finding reveals how lipid rafts create specialized channels capable of moving ions at unprecedented millisecond speeds - far faster than conventional models predicted.
The study, published in this week's edition of Nature Cell Biology, fundamentally challenges our understanding of cellular communication. For decades, scientists believed ion transport occurred primarily through protein channels like ion pumps and voltage-gated pores. The new research demonstrates that lipid rafts - previously considered mere organizational platforms - actually form dynamic quantum tunnels that enable near-instantaneous ion transfer.
What makes this discovery revolutionary is the quantum behavior observed at biological temperatures. "We're seeing quantum coherence effects persisting for milliseconds in these lipid raft channels," explained Dr. Elena Voskresenskaya, lead author from the Max Planck Institute for Biophysical Chemistry. "At 37°C, in salty aqueous environments, this level of quantum behavior was thought to be impossible. Our measurements show clear evidence of quantum tunneling facilitating ion transport."
The research team employed an innovative combination of super-resolution fluorescence microscopy and quantum coherence spectroscopy to track individual ions moving through membrane rafts. Their data revealed distinct "hot spots" where calcium and potassium ions traversed the membrane in 0.3-1.2 millisecond bursts - approximately 100 times faster than through conventional ion channels.
Lipid rafts, those cholesterol-rich microdomains in cell membranes, appear to transiently form what researchers call "quantum vortices" - swirling arrangements of lipids that create temporary conductive pathways. These vortices exhibit remarkable properties: they self-organize, maintain quantum states long enough for ion transfer, and then rapidly dissipate. The entire process resembles a quantum version of a subway system, with ions hopping on and off these fleeting molecular trains.
Medical implications are profound. Many neurological disorders involve ion dysregulation - from epilepsy to migraines to neurodegenerative diseases. The discovery of this ultra-fast transport system suggests we've been missing a major player in cellular signaling. "About 30% of ion traffic in neurons appears to use these quantum raft channels," noted co-author Dr. Rajiv Deshpande from Cambridge University. "This could explain how the brain achieves such rapid signal processing despite relatively slow individual action potentials."
The quantum nature of these channels also solves a long-standing mystery in cardiac physiology. Cardiomyocytes need near-simultaneous calcium signaling across entire cells for coordinated contraction. Classical diffusion models couldn't fully explain the speed and synchronization observed. Quantum raft channels provide the missing mechanism - allowing virtually instantaneous calcium waves that keep the heart beating rhythmically.
From a physics perspective, the findings are equally astonishing. Biological systems were thought too "noisy" and warm to sustain quantum effects beyond femtoseconds. Yet these lipid raft channels maintain quantum states for milliseconds - an eternity in quantum terms. Researchers speculate that the unique molecular arrangement of sphingolipids and cholesterol in rafts creates a protective environment that shields quantum states from decoherence.
Technological applications are already being envisioned. The team's quantum coherence measurements suggest these biological structures could inspire new types of quantum computing components that operate at room temperature. "Nature has solved problems we're still struggling with in quantum engineering," remarked Dr. Voskresenskaya. "These lipid rafts are essentially biological quantum devices that have evolved over billions of years."
The discovery also raises intriguing evolutionary questions. If quantum effects play such a fundamental role in basic cellular processes, might they contribute to consciousness itself? Some theorists have proposed quantum mechanisms in brain function, but lacked plausible biological structures. Quantum raft channels provide concrete pathways for such phenomena.
Next steps involve mapping the full range of ions transported through these quantum channels and developing tools to selectively modulate their activity. Pharmaceutical companies are particularly interested in developing raft-channel modulators that could treat conditions like arrhythmias without affecting other ion channels.
As with any paradigm-shifting discovery, skepticism remains. Some researchers caution that while the data is compelling, more evidence is needed to confirm quantum effects at physiological temperatures. Several labs worldwide are already racing to replicate the findings using alternative methods.
One thing is certain: our understanding of the cell membrane has been forever changed. No longer just a passive barrier or platform for proteins, the membrane emerges as an active participant in cellular signaling - one that harnesses quantum physics to achieve remarkable speed and efficiency. The "quantum express" system in lipid rafts represents one of the most exciting intersections of biology and physics in decades, with implications spanning from medicine to quantum computing.
The full implications will take years to unravel, but this discovery undoubtedly opens a new chapter in our understanding of life at the molecular level. As research continues, we may find that quantum effects in biology are far more prevalent than anyone imagined - with lipid rafts just the first stop on this remarkable scientific journey.

By /Aug 7, 2025
By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 18, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025
By /Aug 7, 2025
By /Aug 18, 2025
By /Aug 18, 2025

By /Aug 7, 2025

By /Aug 18, 2025
By /Aug 7, 2025
By /Aug 18, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025

By /Aug 7, 2025